Shanghai Zimai Safety Technology Co.Ltd,. founded in Shanghai, China, based on over 20 years of export experience in PPE field, ZIMAI innovatively R&D new line of medical devices from year 2021.
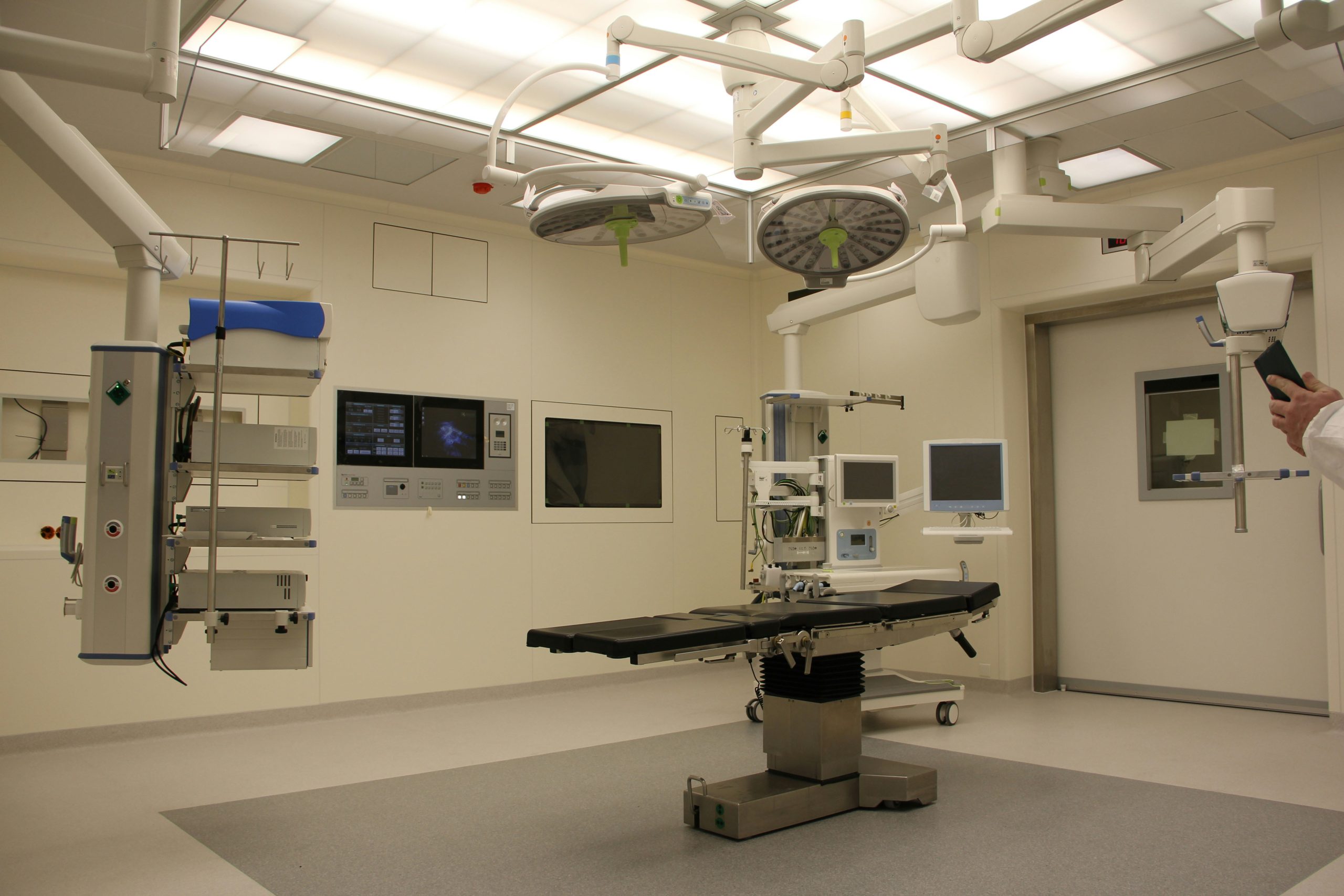
Being a professional supplier of Medical devices, ZIMAI not only provides one-stop purchase services to all the hospital or organizations from OR & ICU Solutions/ Hospital Furniture to Medical Consumables, but also provide the products to all the customers or families from Healthcare Equipment to Emergency Products. ZIMAI dedicates its effort in product certification, which leads the result that most products are CE or FDA certified.
ZIMAI values customers the most, and takes quality as the priority. A quality Management System has already been established to provide a framework for measuring and improving the company’s performance. The company has been certified to ISO 9001:2015 & ISO 14001:2015 and ISO13485:2016, and obtained the medical devices business license in category Ⅱ/Ⅲ.
ZIMAI will always be committed to the filed of medical devices and dedicated to improving the individual heath of mankind!
 Professional medical equipment supplier
Professional medical equipment supplier